They say good things come to those who wait; we say good things come to those who wait—but only those things left by the visionaries before them. The pharmaceutical industry has long been known to lag in the adoption of new technology due to strong regulations. Even though regulators have recently begun to open up to several new ‘big data’ or pharma 4.0 technologies – the question remains: are pharma companies are doing the same? In 2016, the European Pharmacopoeia 9.0 in Chapter 5.21 announced that neural networks (NN) and support vector machines (SVM) are valid chemometric techniques for processing analytical data sets. This is a very encouraging announcement from regulators because, finally, they are providing guidance for the use of chemometrics (the science of extracting information from chemical systems by data-driven means). This alone should empower skeptics to start exploring the magnitude of advantages these techniques can provide due to the dramatic advances of new technologies.
In the past, artificial intelligence (AI) was a subject mostly reserved for academics and researchers, but with time, industries have evolved and have grown interested in these technologies. With the democratization of technology, some topics like AI, machine learning (ML), and cloud platforms have become more of a ‘hype topic’, a trendy concept resulting in a lot of people talking about something but only a few actually understanding and implementing it correctly. Nowadays, AI can be found everywhere – in beer commercials and even commercials for golf clubs. Did you ever think beer companies would have commercials promoting their use of AI to make their product better? Beyond these “hype topics”, there is a lack of understanding of these technologies, and, as a result, there are a lot of people who are hesitant to use them. They see AI as a ‘black box’, which stems from the fact that it is extremely complex and not always understood, just like most of us do not understand how our iPhones are made or how they work (from a technical standpoint). However, this misunderstanding, or more precisely the lack of it, does not help in highly regulated environments where everything has to be accounted for, it just adds more fear of regulatory exposure.
After the announcement by the European Pharmacopoeia and the increasingly relevant need to start improving efficiency in the pharma industry, there are no more scientific excuses for not embracing AI/ML technology. People need to change their mindset and embrace a practical approach to incorporate these powerful AI tools and the compelling benefit they represent. And maybe a key for this change in mindset is just a matter of understanding the capabilities of these technologies better and how they work, to ultimately be able to verify AI models in agreement with regulators.
A few examples are neural networks and support vector machines. At first, it seems difficult to track data and understand how it predicts or classifies, but after the pharmacopoeia’s validation, companies should be investing in understanding it better and establishing methodologies to verify AI models. Using these techniques, and the power of cloud computing, we are essentially trading off ‘interpretability’ for increased precision in predictive analytical models. We believe learning how to verify AI models is the key to solving the ‘chicken vs. egg’ argument – at the nexus between regulators, industry, and vendors.
How to verify AI models
The basics of verifying AI models involve preparing a set of experiments with ‘training data’ and ‘verification data’ and keeping the data acquisition and the AI model under control at all times or documenting compliant conditions.
Preparing a design-of-experiment to verify AI models may require huge computational power, but running them could require less. Currently, Aizon is working on a scientific paper with procedures to verify AI models.
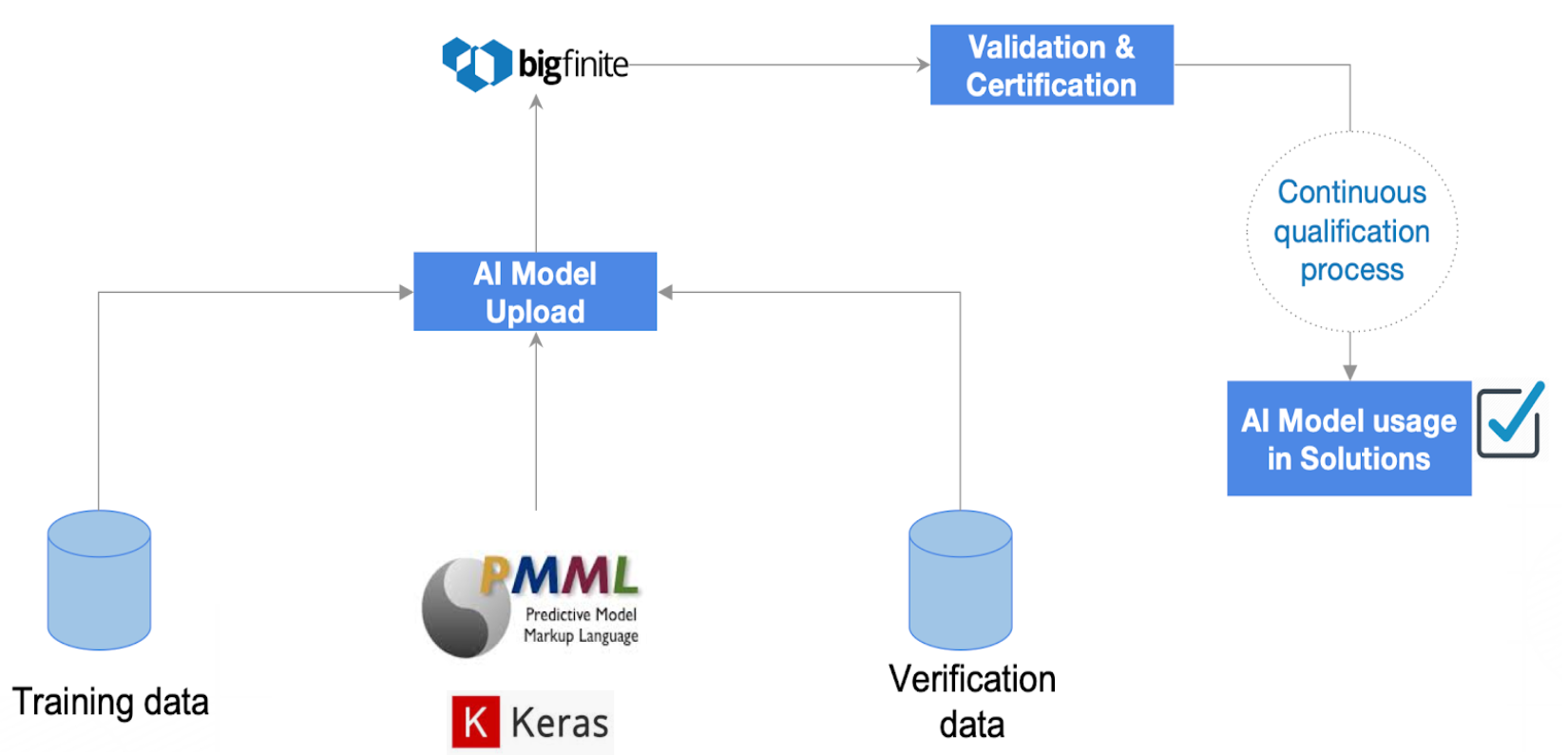
That flexibility on computational power demand is better managed with cloud server-less technology (or on-demand computing). That is technically feasible and already used in other industries, the challenge for the pharma industry is to ensure compliance during the whole process.
Connecting the dots
Today, two out of three points are connected, to advance the realization of benefits from state-of-the-art technologies in pharma and biotech:
- Official regulatory support (European Pharmacopoeia) for the use of AI models as valid chemometric techniques for processing analytical data sets
- Modern, scalable technology to ensure data integrity in the cloud
- People’s mindset, regarding what is possible today, and how to get started
The last point is a broader topic in itself, and warrants an extensive separate post – so stay tuned. At Aizon, we work with curious and courageous market leaders who are not satisfied with following in the steps of others. Contact us today if you are also curious to learn more and innovate your path forward.



